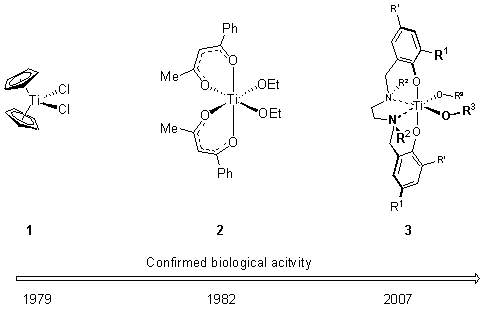
Titanium offers, in contrast to the heavy metals, a low intrinsic toxicity. This together with a rich coordination chemistry makes it an interesting target for studying its bioactivity. The field of bioactive titanium complexes is mainly governed by three different structural types: derivatives of titanocene dichloride 1, diketonato- complexes 2, and salane complexes 3.


Scheme 1 Fluorescence-activated cell sorting (FACS) allows for the differentiation of apoptotic (lower right quadrant) vs. necrotic (upper right quadrant) cells.
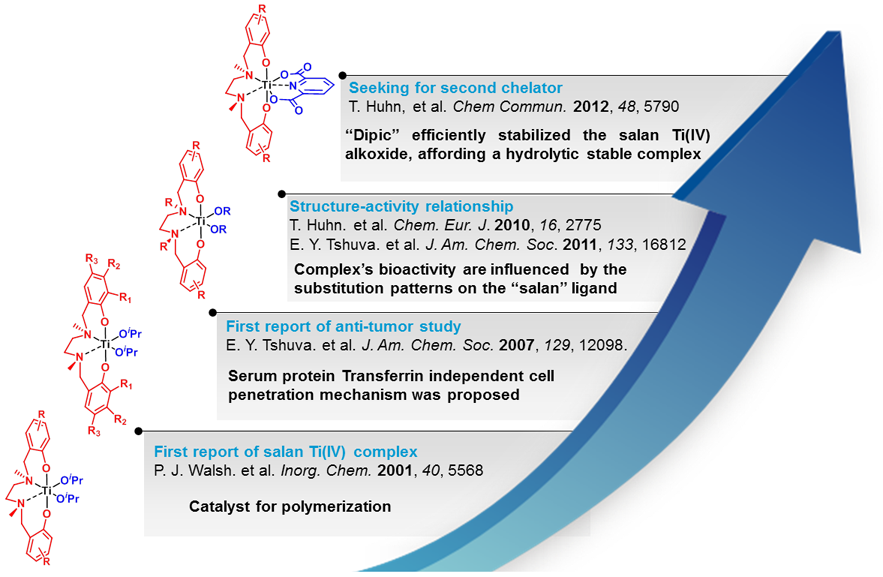
Time resolved 1H-NMR-studies at our in-house facilities revealed the hydrolytic stability of selected complexes. Together with data from cell assays done in our biolab this allowed us a deeper insight in structure-activity relations as well as the identification of compounds with promising pharmacological profile.[3] Those candidates are currently under investigation in a tumor mouse model in collaboration with Prof. Peter Öhlschläger (Department of Biology).
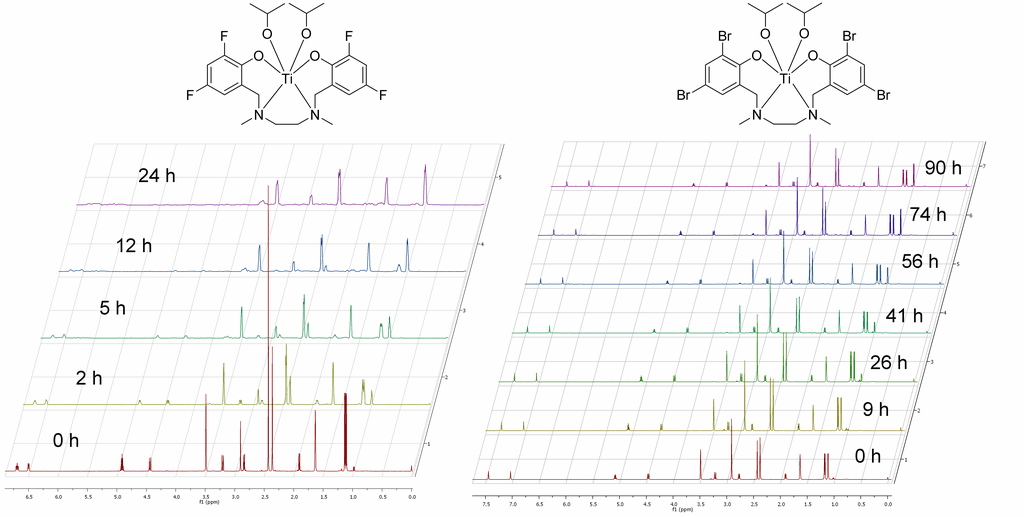
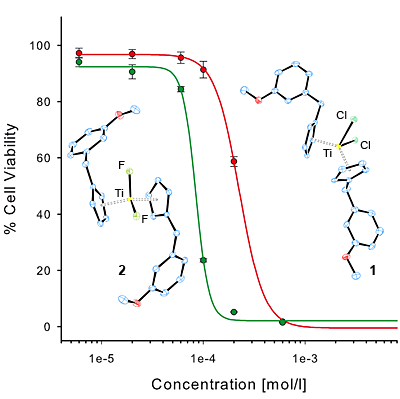
Titanocene dichlorides are known to be prone to hydrolysis. By modification of the labile ligand we recently succeeded in the preparation of rather robust complexes with enhanced hydrolytic stability and cytotoxicity. Exchange of the chloro with the fluoro-ligand in titanocene Y (1) led already to enhanced stability,[2] the methylation of 1 and the concomitant exchange reaction of 3 with different bidentates devised a metal-free access to further stabilized derivatives with an enhanced cytotoxic profile.[4]
11. Cytotoxic Heteroleptic Heptacoordinate Salan Zirconium(IV)-bis-Chelates – Synthesis, Aqueous Stability and X-ray Structure Analysis
F. Schneider, T. Zhao, T. Huhn
Chem. Commun. 2016, 52 (66), 10151-10154.
10. Differential cytotoxicity induced by the Titanium(IV)Salan complex Tc52 in G2-phase independent of DNA damage
T. Pesch, H. Schuhwerk, P. Wyrsch, T. Immel, W. Dirks, A. Bürkle, T. Huhn, S. Beneke
BMC Cancer2016, 16:469.
9. Synthesis and X-ray Structure Analysis of Cytotoxic Heptacoordinate Sulfonamide Salan Titanium(IV)-bis-Chelates
T. Zhao, M. Grützke, K.H. Götz, T. Druzhenko, T. Huhn
Dalton Trans. 2015, 44 (37), 16475 - 16485.
8. Heptacoordinate Heteroleptic Salan (ONNO) and Thiosalan (OSSO) Titanium(IV) Complexes: Investigation of Stability and Cytotoxicity
M. Grützke, T. Zhao, T.A. Immel, T. Huhn
Inorg. Chem. 2015, 54 (14), 6697-6706.
7. Synthesis and X-ray structure analysis of a heptacoordinate titanium(IV)-bis-chelate with enhanced in vivo antitumor efficacy
T. A. Immel, M. Grützke, A.-K. Späte, U. Groth, P. Öhlschläger, T. Huhn
Chem. Commun. 2012, 48, 5790 - 5792.
6. Cytotoxic Dinuclear Titanium-Salan Complexes: Structural and Biological Characterization
T. A. Immel, M. Grützke, E. Batroff, U. Groth, T. Huhn
J. Inorg. Biochem. 2012, 68-75.
5. Titanium Salan Complexes Displays Strong Antitumor Properties In Vitro and In Vivo in Mice
T. A. Immel, U. Groth, Th. Huhn, P. Öhlschläger
PLoS ONE 6(3): e17869 2011.
4. Dimethyl Titanocene Y: a Valuable Precursor for Libraries of Cytotoxic Titanocene Derivatives
T.A. Immel, J.T. Martin, C.J. Dürr, U. Groth, T. Huhn
J. Inorg. Biochem. 2010, 863 - 867.
3. Cytotoxic Titanium Salan Complexes: Surprising Interaction of Salan and Alkoxy Ligands
T.A. Immel, U. Groth, T. Huhn
Chem. Eur. J. 2010, 2010, 2775-2789.
2. Titanocene Difluorides with Improved Cytotoxic Activity
S. Eger, T.A. Immel, J. Claffey, H. Müller-Bunz, M. Tacke, U. Groth, T. Huhn
Inorg. Chem. 2010, 2010, 1292-1294.
1. Highly Selective Apoptotic Cell Death Induced by Halo-Salane Titanium Complexes
T.A. Immel, M. Debiak, U. Groth, A. Bürkle, T. Huhn
ChemMedChem 2009, 738-741.