Our research in the field of molecular electronics is strongly connected to the interdisciplinary cooperation with the Department of Physics at the University of Konstanz. Our group is part of the SFB767 Controlled Nanosystems, that deals with actual and upcoming challenges of nanotechnology. This joint research initiative addresses the challenge of achieving a detailed understanding of the interaction of nanostructures, both among themselves and with their macroscopic environment. The common goal is always to precisely control and manipulate their properties in order to pave the way for new functionalities.
Our research adresses currently three main issues:
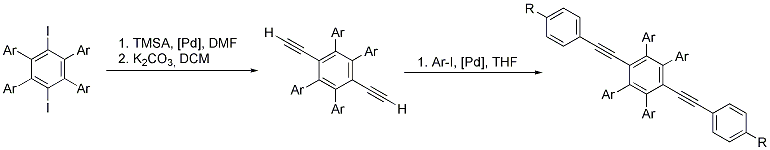
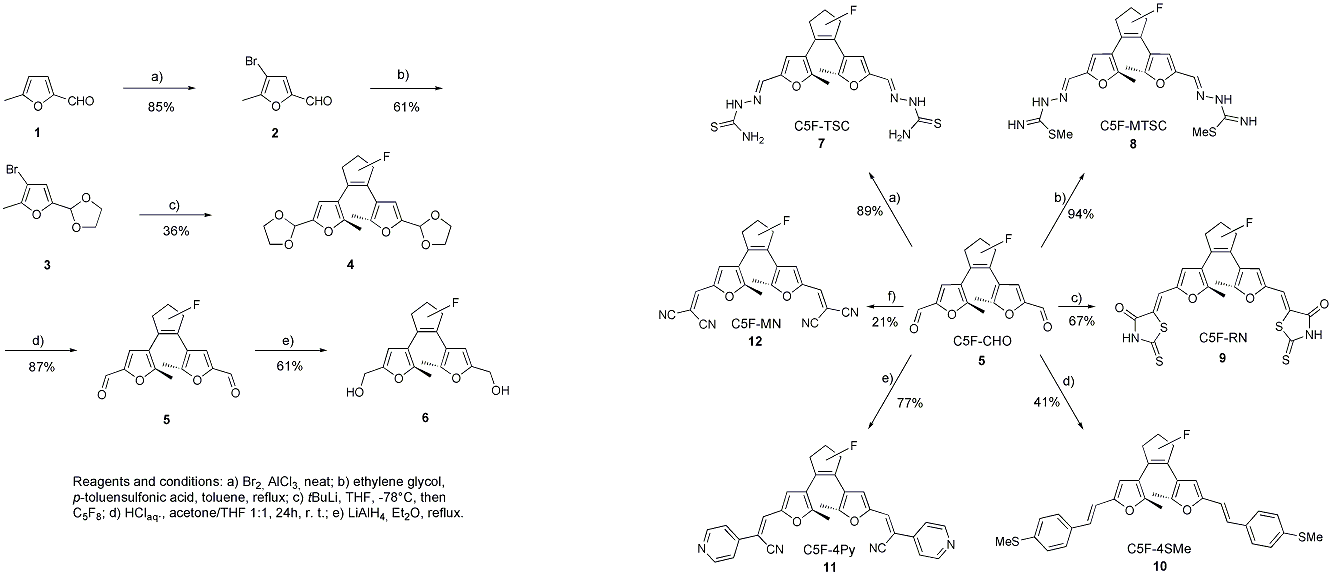
In this field of research, we focus on the synthesis of photoswitchable molecular wires. Irie-type molecular switches have gained quite a lot of interest during the last few years. One of the latest results was the single-molecule conductance of pyridine-terminated dithienylethene molecules by Tam et al. However, mostly all of the recent experiments were carried out by the community with dithienylethene-based chromophores. Since the central switching unit bears sulfur atoms, which bond to gold, the interpretation of gained transport measurement data might be troublesome and misleading. Different binding modes to the electrodes are bypassed using difurylethene based chromophores. We developed a convenient and high yielding route to the furaldehyde 5, which is shown in Scheme 1.[1]

This extremely useful intermediate is smoothly transformed to molecules with various anchoring groups at both ends that bind to gold. With the help of the π-system attached to the chromophore, we were able to trim the switching properties in the desired way. We do photokinetic analysis of the switching process with our UV/Vis equipment in the work group (Scheme 2.)[1]
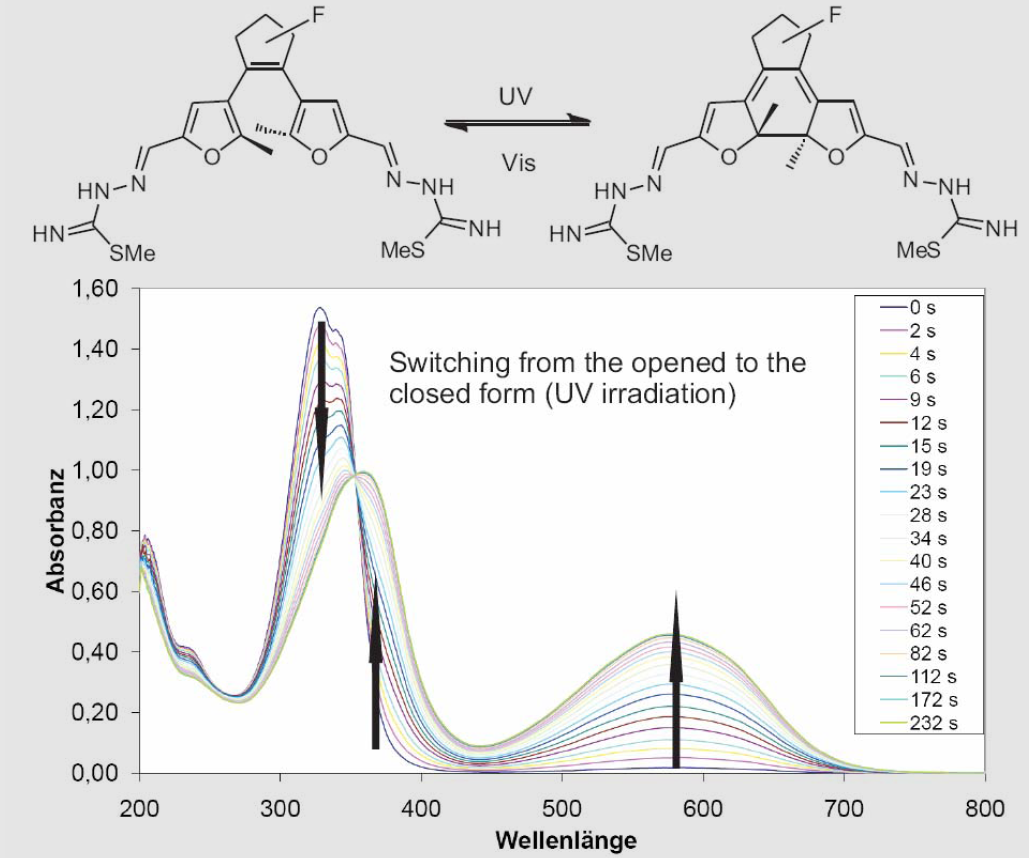
Scheme 2. UV-spectra of the switching process.
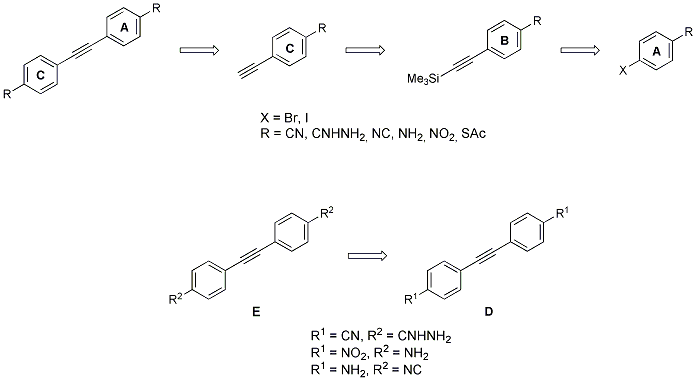
Scheme 3. Synthetic approach towards OPEs with different anchoring groups via Sonogashira coupling.
We demonstrated that different anchoring groups influence the charge transport properties of the molecules in a single molecule transport experiment Scheme 4.[1] The experiments were carried out in a mechanically controlled break junction (MCBJ) in a liquid cell at the Department of Physics by our cooperation partner LS Scheer/Dept. Physics .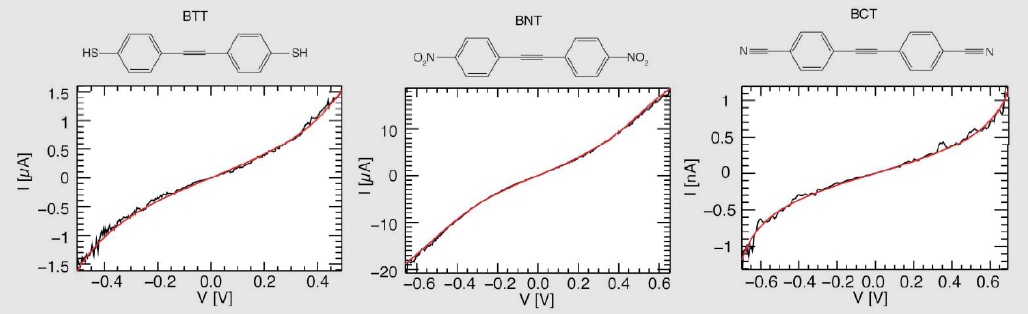
In this field of our research, we aim to modify to electronic properties of oligo-phenylene-ethynylenes (OPE) by means of organic synthesis. This class of molecular wires is well known, and their conductivity was demonstrated in STM experiments. Since the electronic properties are linked to the transmission function of a molecule, we tend to tune the charge transport by tailoring functional moieties along the conductor path.
One class of OPEs we are working on is the class of 2,3,5,6-substituted 1,4-bis-phenylene-ethinylene-benzenes. The central benzene ring bearing the donating moieties is built up in a one-pot multi-aryne-sequence Scheme 5. Various electron donating or withdrawing functional groups are introduced smoothly with this reaction.
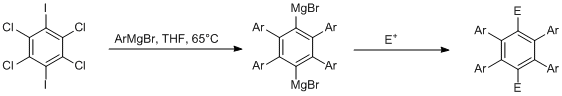
The obtained arylbenzenes were transformed into OPEs by Sonogashira cross-coupling according to Scheme 6. Coupling with trimethylsilylacetylene,followed by deprotection, gave the corresponding bis-ethinylbenzenes. Those were coupled with 4-functionalized iodobenzenes.